
This federal standard allows plasma donation centers to operate efficiently with no impact on safety for either donors or recipients of donated plasma. Food and Drug Administration’s (FDA) plasma donation regulations. For example, California requires licensed medical professionals to perform some of the necessary tests on potential plasma donors, while the FDA requires appropriately trained, but not necessarily licensed, professionals to perform these tests (note that Governor Newsom recently signed a bill to bring CA into alignment with federal standards). Regulation of plasma donation centers falls to states, and some states have enacted regulations that exceed the U.S.
Aligning state plasma donation regulations with federal standards With demand for source plasma steadily rising, removing unnecessary or unintentional barriers to plasma donation is critical. Only source plasma from volunteer donors can be used to create these life-saving medicines. Unfortunately, there are no synthetic substitutes for plasma-derived therapies. These therapies include immunoglobulin used by those with primary immunodeficiency and autoimmune disorders, clotting factors for those with hemophilia, and albumin used for burn and trauma victims. Why plasma donation access and awareness mattersĪ consistent supply of source plasma from donors is critical to maintaining the supply of plasma-derived therapies used by hundreds of thousands of individuals around the world. Increase policymaker and public awareness of the need for plasma donation.Address barriers that prevent plasma donation, such as state regulations that do not align with federal standards and border control measures that adversely affect the ability of non-U.S.IDF works to ensure adequate source plasma supply on two fronts.

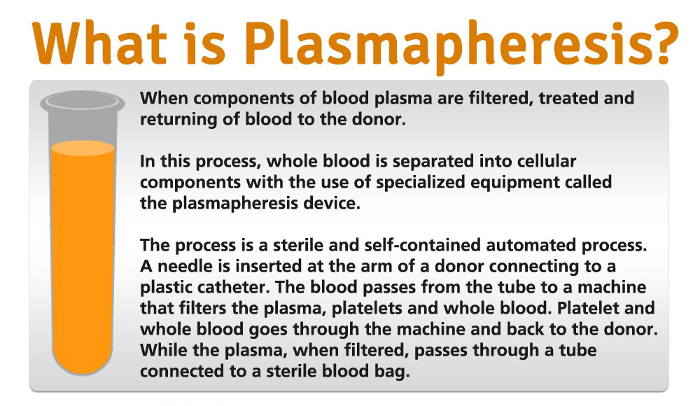
Starting in late 2018 and continuing through the COVID-19 pandemic, there has been concern about potential immunoglobulin shortages due to increasing demand for plasma-based therapeutics and a decline in overall blood plasma donation. It takes approximately 130 plasma donations to produce one year’s supply of immunoglobulin for an adult with PI. If you experience these, contact the CSL Plasma center.Many patients with primary immunodeficiency (PI) rely on plasma-based therapeutics (namely, immunoglobulin) that cannot be manufactured without the donation of source plasma - the liquid, cell-free component of blood - by volunteers. Signs of infection include pain, swelling, or feeling of warmth at the site of the needle insertion. Donating plasma comes with a slight risk of infection. If you’re uncomfortable, you can speak with a clinician. Bruising may occur at the site of the needle insertion, and you could experience some discomfort during the donation process. If you experience dehydration or electrolyte imbalances, you may also experience tiredness after donating plasma. Donating plasma may cause mild electrolyte imbalances because plasma contains a lot of salts, vitamins, and minerals (electrolytes) that help your body’s functions. Because plasma contains water, removing it may cause donors to experience mild dehydration after donation. Short-term plasma donation side effects include:

For healthy adults who meet the donation eligibility requirements, donating plasma is generally a safe medical procedure, however you may face mild immediate side effects.


 0 kommentar(er)
0 kommentar(er)
